Social restrictions could start to be eased by spring, one scientist has said, after the University of Oxford and AstraZeneca published their early trial data for their Covid vaccine.
AstraZeneca and Oxford University announced that their jab is effective in preventing many people getting ill and it has been shown to work in different age groups, including the elderly.
One Sage scientist said recent vaccine news – including the development from Oxford – could mean that social restrictions could start to be eased by spring.
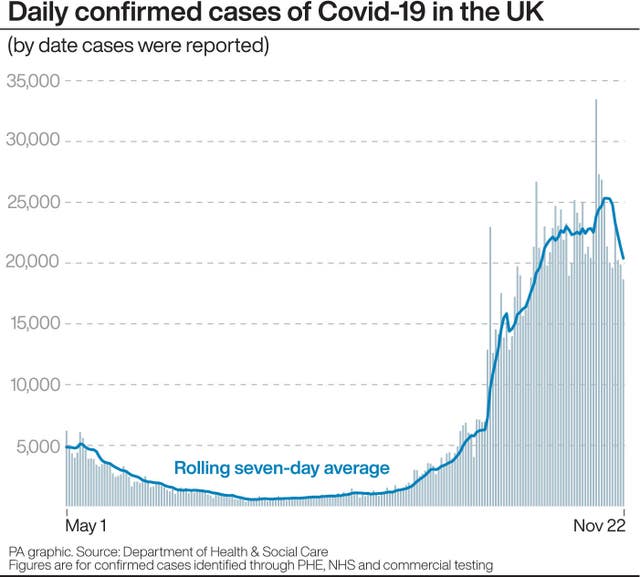
But he said that people need to keep up with protective measures, including social distancing.
Dr Michael Tildesley, associate professor in infectious disease modelling at the University of Warwick, told Times Radio the news was “excellent” but said it could be four to five months before enough vaccine could be given to the population to achieve some sort of herd immunity.
“It’s absolutely excellent news about the Oxford vaccine because this is really the vaccine that the Government has pinned a lot of their hopes on in terms of resources – we’ve ordered 100 million doses of the Oxford vaccine,” he said.
“The vaccine is on the horizon. But we’re still probably four/five months away from getting to the stage that we can give enough doses out to the population to start thinking about achieving herd immunity as a nation.
“What we really need to do in the meantime is get the message out there that, yes, there is good news – the cavalry is appearing on the horizon – but we need to keep incidence as low as possible. We need to keep observing our social distancing.”
When asked whether by summer next year the world would be looking back at the pandemic, he said: “I hope that’s the case. I would say I was more concerned about it four or five weeks ago when it was looking to me that we might still be in the situation next summer.
“We’ve had some great news about three different vaccines over the last three weeks.
“I would say I’m more hopeful that by the spring we might be starting to ease out of these restrictions.”
Other experts welcomed the news that the vaccine can be delivered around the world using existing healthcare facilities.
The vaccine can be stored in “fridge temperature” from 2-8C.
Professor Azra Ghani, chair in infectious disease epidemiology at Imperial College London, said: “The results from this trial of the Oxford/AZ vaccine are highly encouraging, demonstrating significant efficacy.
“A particular strength of this vaccine is that it can be stored in a fridge; this means that it can be distributed around the world using existing delivery mechanisms.
“This could therefore have a truly significant impact across the globe and enable an end to the Covid-19 pandemic.”

On the efficacy rates compared to other vaccines, she added: “Of course much will be made of the difference in overall efficacy between this vaccine (70%) and the Pfizer and Moderna vaccines (95%).
“However, it is encouraging to see that in a sub-analysis, a fractional dosing schedule in which the first dose was administered at a lower level than the second resulted in higher efficacy and gave results comparable to the other vaccines (90%).
“It will be important to both understand the mechanisms generating this result as well as to confirm this in the final efficacy analysis.
“If this result holds, this will mean that the same manufacturing capacity will be able to produce more doses and thus further reduce the constraints on vaccine supply.”
Peter Horby, professor of emerging infectious diseases and global health in the Nuffield Department of Medicine at the University of Oxford, added: “This is very welcome news, we can clearly see the end of the tunnel now.
“Importantly, from what we have heard, the vaccine seems to prevent infection, not just disease. This is important as the vaccine could reduce the spread of the virus as well as protect the vulnerable from severe disease.
“The Oxford vaccine can be stored in the fridge, as opposed to the freezer like the other two vaccines, which means it is a more practical solution for use worldwide.”
Dr Michael Head, senior research fellow in Global Health at the University of Southampton, said: “This vaccine candidate also requires refrigeration storage rather than the ultra-low temperatures of the Pfizer candidate.
“Oxford have previously confirmed that there would be some level of distribution to low-and-middle-income countries so this may also be good news around the subject of equitable access to vaccine development with a product that is much easier to store and distribute.
“The pandemic is everyone’s problem at least until the vast majority of the globe is vaccinated, not just the rich countries.”
Professor Brian Cox said the speed of vaccine development was “breath-taking”.
He wrote on Twitter: “The speed of development of vaccines has been breathtaking – and whilst it shouldn’t really need saying, demonstrates the almost incalculable value of a strong university sector and wider science base.”
The speed of development of vaccines has been breathtaking – and whilst it shouldn’t really need saying, demonstrates the almost incalculable value of a strong University sector and wider science base. Thank you to the researchers from post docs to Profs who did this work. https://t.co/AiwON4XWeu
— Brian Cox (@ProfBrianCox) November 23, 2020
Dr Charlie Weller, head of vaccines at Wellcome, said the preliminary results were “hugely encouraging”.
She said: “These results suggest it is highly effective in protecting serious illness and it may reduce transmission.
“It is based on an established vaccine technology, which does not require the challenging cold-chains and should therefore ease deployment and global access.
“As with all interim results we have seen, it is critically important that the trial is completed and regulators can now independently and rigorously assess the data.
“Vaccines, treatments and tests are all vital to protect lives and end this pandemic as soon as possible.
“To have interim results from three vaccine teams within a year is incredible and testament to a truly extraordinary global scientific effort.
“We need a range of vaccines, that can protect people of different ages and backgrounds wherever they live, and to be able to manufacture enough doses for the world.”
Professor Dame Ottoline Leyser, chief executive of UK Research and Innovation, which helped to fund the vaccine, added: “It’s a Herculean achievement in under a year and a tribute to the dedication of many people – from scientists and clinicians in universities and industry to the trial volunteers – who have come together to deliver this promising vaccine with tremendous speed.
“These preliminary phase three results show the Oxford-led Covid-19 vaccine could be more effective against coronavirus than typical vaccines against seasonal flu, but more study is needed to understand dosing and the protective response from the vaccine.”
Eleanor Riley, professor of immunology and infectious disease at the University of Edinburgh, said the trial has the potential to answer questions about transmission.
She said: “If there is anyone out there who still doubts that a Covid-19 vaccine can help us out of this pandemic, the announcement today from Oxford University/Astra Zeneca should surely dispel that doubt.
“This is excellent news – another vaccine that can prevent symptomatic infections and, even better, is cheap to produce and easy to distribute.
“This trial also has the potential to answer questions about virus transmission. In contrast to some other trials, the Oxford/Astra Zeneca team has been collecting weekly nasal and throat swabs from all trial participants to look for asymptomatic infections.
“If the vaccine reduces transmission – ie, vaccinated individuals have fewer asymptomatic infections, or their viral load is lower if they are infected, or if they shed virus for a shorter period of time – the vaccine could make an important contribution to herd immunity.”
Dr Richard Hatchett, chief executive of the Coalition for Epidemic Preparedness Innovations (CEPI) said: “We believe that this vaccine candidate has the potential to significantly alter the course of the global pandemic.
“The data released today suggest the vaccine is safe and comparable in its efficacy to other licensed vaccines – including influenza – that are widely used to protect people around the world today.
“AstraZeneca and Oxford have developed an affordable, scalable vaccine that crucially can be stored and shipped in a regular refrigerator. This makes it appropriate for use and easy to deliver almost anywhere in the world, including in low-resource settings.
“That means that this vaccine will make an important contribution to controlling the pandemic globally.”
Prof Peter Piot, director of the London School of Hygiene & Tropical Medicine, added: “2020 will be remembered for the many lives lost from Covid-19, lockdowns and the US election. Science should now be added to this list.
“Breakthroughs in science are nothing new, but the importance of the three announcements this month from Pfizer/Biontech, Moderna, and today from University of Oxford-AstraZeneca, cannot be overestimated.
“November 2020 looks set to be the month that humanity developed the tools to turn the tide against this devastating virus.”




Comments: Our rules
We want our comments to be a lively and valuable part of our community - a place where readers can debate and engage with the most important local issues. The ability to comment on our stories is a privilege, not a right, however, and that privilege may be withdrawn if it is abused or misused.
Please report any comments that break our rules.
Read the rules here